History
The earliest reported cases in the US began appearing in late March 2009, in California,[119][120] then spread to infect people in Texas, New York, and assorted other states by mid-April.
On April 22, the CDC first activated its Emergency Operations Center (EOC). On April 25, the World Health Organization (WHO) declared a public health emergency of international concern.
The disease then spread across the country’s population and by the end of May had infected citizens in all 50 states. The pattern continued through June of the same year. The total number of confirmed cases varied from 27,717[121] (Centers for Disease Control and Prevention (CDC) confirmed and probable cases) and 25,453 (total of all state confirmed cases) as of June 26, 2009.
Towards the middle of June 2009, the number of US cases surpassed those of Mexico, which had been the previous leader in diagnosed cases of the disease. Toward the end of June 2009, the number of deaths related to the virus in the US surpassed those of all other countries as well.
On June 25, the CDC released information revealing that there were more than likely over one million (1,000,000) cases of the disease in the US, most of which had not been reported or diagnosed.[122][123]
Deaths relating to this new strain of influenza began appearing in the US in late April, and by early June 15, states had reported fatalities related to or directly occurring from the virus. These deaths totaled at 4,000 as of November 15, 2009. The CDC distributed a vaccine for the novel flu strain.[124]using mechanisms already in place for its Vaccines for Children (VFC) program.[125]
On April 26, 2009, the U.S. Department of Health and Human Services declared Swine Flu a public health emergency. [126] On October 24, 2009, President Barack Obama declared Swine Flu a national emergency in the United States. On November 12, 2009, the CDC reported an estimated 22 million Americans had been infected with 2009 A H1N1 and 4,000 Americans have died.[127] On December 10, 2009, the CDC reported an estimated 50 million Americans or 1 in 6 people had been infected with the 2009 A H1N1 Virus and 10,000 Americans had died, by which time the vaccine was beginning to be widely distributed to the general public by several states.[128] On December 23, 2009 the CDC reported a reduction of the disease by 59% percent and the disease was expected to end in the United States in January 2010.
On January 15, 2010, the CDC released new estimate figures for swine flu, saying it has sickened about 55 million Americans and killed about 11,160 from April through mid-December.[129] On February 12, 2010, the CDC released updated estimate figures for swine flu, reporting that, in total, 57 million Americans had been sickened, 257,000 had been hospitalised and 11,690 people had died (including 1,180 children) due to swine flu from April through to mid-January.[130]
Initial cases[edit]
The Centers for Disease Control and Prevention (CDC) identified the first two A/09(H1N1) swine flu cases in California on April 17, 2009, via the Border Infectious Disease Program,[131] for the San Diego County child, and a naval research facility studying a special diagnostic test, where influenza sample from the child from Imperial County was tested.[132] By April 21, enhanced surveillance was established to search for additional cases in both California and Texas and the CDC determined that the virus strain was genetically similar to the previously known A(H1N1) swine flu circulating among pigs in the United States since about 1999.
It was established that the virus was a combination of human, North American swine, and Eurasian swine influenza viruses; the viruses from the initial two Californian cases were also noted to be resistant to amantadine and rimantadine, two common influenza antiviral drugs.[133] No contact with pigs was found for any of the seven Californian nor either of the two Texas cases, suggesting human-to-human transmission of the virus.
On April 28, 2009, the director of the Centers for Disease Control and Prevention confirmed the first official US death of swine flu. Tests confirmed that a 23-month-old toddler from Mexico, who was probably infected there, died on April 27 from the flu while visiting Texas.[134]
Outbreak across the US[edit]
![[icon]](https://upload.wikimedia.org/wikipedia/commons/thumb/1/1c/Wiki_letter_w_cropped.svg/20px-Wiki_letter_w_cropped.svg.png) |
This section needs expansion. You can help by adding to it.(December 2009) |
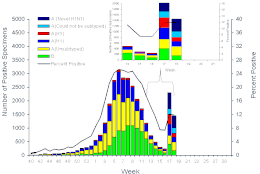
CDC report for the 2008-2009 flu season week 18 (May 17), subtypes and percent positive tests
Cases of H1N1 spread rapidly across the United States, with particularly severe outbreaks in Texas, New York, Utah, and California. Early cases were associated with recent travel to Mexico; many were students who had traveled to Mexico for Spring Break.[135] On May 4, 2009, the CDC reported one death, 286 confirmed cases of H1N1 flu across 36 states, 35 hospitalizations, and expects H1N1 to eventually spread to all states. A large number of cases, according to medics, have happened in the days that preceded the launch of the alert and came out only in these days due to a massive backlog.[136] By May 5, 2009, the number had risen to 403[121] and a second death was reported in Texas.[137] The CDC and government officials had expressed cautious optimism about the severity and spread of H1N1.[138][139]
Changes in surveillance of cases of influenza-like illness, including new guidelines for identifying cases to test, increased laboratory testing, and new test kits able to distinguish this novel strain, resulted in a spike in the percent of cases tested positive for influenza. Of the positive cases, about a third were due to the novel strain. Also found were a substantial number of cases where the strain could not be subtyped.[140]

Pneumonia and influenza deaths in 122 US cities, 5 years through October 2009
The proportion of US deaths due to pneumonia and influenza climbed above the epidemic threshold in the 2007–2008 winter flu season but not in the 2008–2009 season. Although the 2009 H1N1 outbreak reached epidemic levels of infection early in 2009, it did not contribute to epidemic levels of pneumonia and influenza related deaths until October 2009.[citation needed]
| 2009 US Swine Flu Summary |
|
| Number of Confirmed Cases/Deaths |
See Table Above |
Number of States/Territories
with Reported Cases |
56 |
Number of States/Territories
with Confirmed Deaths |
55 |
| Earliest Confirmed Infection in US |
March 28, 2009[141] |
| First Death Inside the US |
April 27, 2009[134] |
| First Death of US Citizen |
May 5, 2009[142] |
| Number of People Hospitalized |
9,079 (as of September 3)[143] |
| Fatalities |
593 (as of September 3)[143] |
The second wave[edit]
![[icon]](https://upload.wikimedia.org/wikipedia/commons/thumb/1/1c/Wiki_letter_w_cropped.svg/20px-Wiki_letter_w_cropped.svg.png) |
This section needs expansion. You can help by adding to it.(December 2009) |
|
|
This section’s factual accuracy may be compromised due to out-of-date information. Please update this article to reflect recent events or newly available information. (November 2010) |
In early October 2009, the Centers for Disease Control and Prevention announced that swine flu was widespread across the country. It also said there was significant flu activity in virtually all states, which was considered to be quite unusual for this time of year. There was particular worry about pregnant women. As of late August, 100 had been hospitalized in intensive care units and 28 had died since the beginning of the outbreak in April. On October 1, it was acknowledged that a recruit in basic training in Fort Jackson, South Carolina, was the Army’s first swine flu death. The recruit fell ill on September 1 and died of pneumonia on September 10.
Dell Children’s Medical Center in Austin, Texas, erected two tents in its parking lot to handle emergency room visits, and hospitals around Colorado Springs recorded a 30 percent spike in flu visits. As pediatric cases were increasing, the Dept. of Health and Human Services released 300,000 courses of children’s liquid Tamiflu from the national pandemic stockpile in late September, with the first batches sent to Texas and Colorado.
In late September, the disease centers reported that 936 had died of flu symptoms or of flu-associated pneumonia since August 30, when it began a new count of deaths, including some without laboratory-confirmed swine flu.[144]
The Agriculture Department reported on October 16 that three pigs at the Minnesota State Fair, in St. Paul, were tested positive in late August for H1N1 virus, which were the first cases in the country, although infected pigs had been found in eight other countries. There were 103 pigs tested at the Fair, including the three infected, though all appeared healthy. Scientists said the virus was already spreading widely among people, and, in fact, was more common in humans than in pigs, so humans were more likely to catch it from others than from pigs.[145]
In mid-October, it was reported that flu caused by the H1N1 virus was widespread in 41 states, and flu-like illnesses accounted for 6.1 percent of all doctor visits, which was considered high[citation needed], particularly for October. Forty-three children had died from H1N1 since August 30, which is approximately the number that usually dies in an entire flu season. Nineteen of the forty-three were teenagers while sixteen were between ages five to eleven. The rest were under five.[citation needed] It is reported that the severity of the disease was not increasing. About fifteen to twenty percent of the patients hospitalized for the flu were placed in the intensive care unit, a level similar to that for seasonal flu.[citation needed]

High-risk groups line up at a defunct Kmart on October 24, 2009 for the first H1N1 vaccines publicly available in Boise, Idaho.
Projections of the supply of H1N1 vaccine had decreased significantly from a level of 120 million doses ready in October, estimated during the summer, to an estimate of 28 to 30 million doses by the end of the month. On October 14, 11.4 million doses of the H1N1 vaccine were said to be available. As of November 20, 2009, the CDC reported sharp declines in H1N1 activity throughout the United States, with influenza-like illness (which may also include meningitis, pneumonia, strep pharyngitis, gastroenteritis, and the common cold) accounting for 5.5% of doctors visits, down sharply from 8% in late October, the peak of the second wave. However, taking the vaccine is still urged by the CDC, as a third wave of the disease may sweep across the US, possibly in January/February 2010.[146] as of December 24, the second wave of H1N1 has clearly peaked, with pneumonia and influenza deaths falling below the epidemic threshold for the first time in 11 weeks, and the proportion of doctors visits do to influenza-like illness falling to baseline (2.3%), down from 5.5% 1 month before, on November 20. However, it was reported that influenza activity was beginning to increase in West Virginia, with 5.2% of patients treated by West Virginia health care providers having influenza-like illness, a major increase from 2% of patients treated by West Virginia health care providers having influenza-like illness in November.
Flu strain severity[edit]

Influenza-associated pediatric deaths reported to CDC, from 2005–06 to start of 2009–2010
The new strain was identified as a combination of several different strains of Influenzavirus A, subtype H1N1, including separate strains of this subtype circulating in humans (see human influenza) and in pigs (see swine influenza). The strain transmits between humans and was initially reported to have a relatively high mortality rate in Mexico. The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) have expressed serious concerns that the new strain has the potential to become an influenza pandemic.[147] It is reported that, because the virus is already widespread, containment will be impossible.[148]
In addition, the flu death toll in Mexico could be lower than first thought, Gerald Evans, head of the Association of Medical Microbiology and Infectious Disease Canada and a member of a federal pandemic-planning committee, said on April 29:[149]
There was a lot of speculation and what seemed to be evidence there were dozens and dozens of deaths. Careful analysis showed these people likely died of something else, and not influenza. That’s really good news, and that would fit with what we’ve seen outside of Mexico.
Moreover, another Canadian expert, Neil Rau, criticized the WHO’s decision to raise its Pandemic alert to level 5, saying:[150]
I don’t agree with (the WHO) because I think it’s a panic metre, not a pandemic metre. […] If that flu-like illness is not deadly, I don’t know what the cause for alarm is for people who are not really sickened by this virus. […] I’m really eager to know how much worse this is than seasonal flu. So far it’s looking like it’s not that serious.
CNN stated on April 28, 2009 that there were at least 800 deaths in the U.S. due to normal influenza in each individual week between January 1 and April 18, which is higher than the combined worldwide death toll for the swine flu.[151]
As of November 19, 2009, the official death toll attributed directly to the novel H1N1 and seasonal influenza was 877.[152] This total exceeds the 849 deaths directly attributed to seasonal influenza in 2006.[153]Many of the other deaths commonly attributed to influenza are caused by complicated influenza, where a second infection causes death, usually pneumonia (of which 48,657 of 55,477 official deaths in 2006 occurred in people aged 65 years and older)[153][154]
Response[edit]

Congruent U.S. Government and WHO Pandemic Response Charts.
The Federal response remained at US Pandemic Stage 0, congruent with the World Health Organization (WHO) Pandemic Phases 1, 2 and 3;[155][156]however, the WHO’s Pandemic Phase was raised to 4 on April 27, which is congruent with US Pandemic Stage 2.[157] On April 29, the WHO raised the pandemic alert level to phase 5.[158]
The United States federal government declared a public health emergency, and several U.S. states then indicated that they may follow suit. Secretary of Homeland Security Janet Napolitano noted that this declaration was standard operating procedure, which was also done for the 2009 presidential inauguration and for flooding.[159]
After many days of deliberation the WHO declared that the current influenza had become a true pandemic, raising the Pandemic Alert level to Phase 6, the highest on the WHO scale and congruent with U.S. Federal Government Response Stages 3–6.[160]
White House[edit]
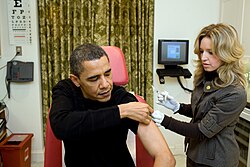
Barack Obama being vaccinated on Dec. 20, 2009.
An official for the White House said on April 24 that “the White House is taking the situation seriously and monitoring for any new developments. The president has been fully briefed.”[161] President Barack Obama stated that “We are closely monitoring the emerging cases of swine flu”. He also noted, “This is obviously a cause for concern … but it is not a cause for alarm”.[162] President Obama suggested that U.S. schools should consider shutting down, as a future possibility, if their students were to become infected.[163] White House Press Secretary, Robert Gibbs said the effort to get a team in place to respond to the health scare has not been hindered by the lack of a secretary of Health and Human Services or appointees in any of the department’s 19 key posts.[164] The president’s nominee, Kansas Gov. Kathleen Sebelius, was still awaiting confirmation from the U.S. Senate until passing on April 28. The President has not yet made appointments to either the Commissioner of the Food and Drug Administration, the Surgeon General, or the Director of the Centers for Disease Control and Prevention.[164] The current acting Surgeon General, Steven K. Galson, is also currently serving as the Acting Assistant Secretary for Health.[165]

Health and Human Services Secretary Kathleen Sebelius in a meeting in the Situation Room of the White House regarding the H1N1 virus.
On April 30, it was reported that an aide to Steven Chu, the US Energy Secretary, had fallen ill from the virus after helping arrange President Barack Obama‘s trip to Mexico.[166] However, the White House stated that the President is not at risk of obtaining the flu.[166] Kathleen Sebelius was confirmed as the Secretary of Health and Human Services by the Senate on April 28, 2009 with a vote of 65–31.[167][168]
On October 24, President Obama declared the 2009 H1N1 swine flu a national emergency.[169] The declaration will make it easier for U.S. medical facilities to handle a surge in flu patients by allowing the waiver of some requirements of Medicare, Medicaid and other federal health insurance programs as needed.
Centers for Disease Control and Prevention (CDC)[edit]
In this video, Joe Bresee, with CDC’s Influenza Division, describes the symptoms of swine flu and warning signs to look for that indicate the need for urgent medical attention.
See also: See this video with subtitles in YouTube
- Activation of Emergency Operations Center
During the week of April 19, 2009, the CDC activated its Emergency Operations Center (EOC), with RADM Stephen Redd as the Incident Commander, to augment the ongoing investigation of human cases of swine influenza A (H1N1).[170] More than 250 CDC professionals are working from the CDC EOC as part of the agency’s response.[171] As of May 4, 2009, the CDC reported that it had deployed 25% of the supplies and medicines in the Strategic National Stockpile to the various states.[172]
- Swine Flu Test Kits
As of April 29, only the CDC could confirm U.S. swine flu cases.[173] Besser stated during an April 30 press briefing that California and New York had diagnostic test kits, and that the kits would be sent to all states starting the following day.[174] On May 6, the CDC announced that testing kits were now available for all states. This is expected to generate an increase in the number of confirmed cases as more states begin doing their own tests.[175]
- Influenza Reporting Requirements
In the United States, the majority of the 70 National Respiratory and Enteric Virus Surveillance System (NREVSS) laboratories do not report the influenza A subtype.
However, in 2007, human infection with a novel influenzavirus A became a nationally notifiable condition. Novel influenza A virus infections include all human infections with influenza A viruses that are different from currently circulating human influenza H1 and H3 viruses. These viruses include those that are subtyped as nonhuman in origin and those that are unsubtypable with standard methods and reagents.[176] The new strain responsible for this outbreak was one such virus.
- CDC Recommendations for Schools
Initially the CDC had issued a recommendation that schools close for as long as two weeks if a student catches swine flu.[177] Some school districts closed all schools if a single child was classified as probable.[135] On May 5 the CDC retracted its advice stating that schools that were closed based on previous CDC guidance related to this outbreak may reopen.[178] By that time at least 726 schools nationwide serving more than 480,000 students had closed for at least some period of time.[177] The CDC amended its advice citing, new information on disease severity and the limiting effectiveness of school closure as a control measure.[178] The new advice given stated, “Decisions about school closure should be at the discretion of local authorities based on local considerations, including public concern and the impact of school absenteeism and staffing shortages.”[178]
Food and Drug Administration[edit]
The Food and Drug Administration (FDA) authorized emergency use of medicines and diagnostic tests for flu. (FDA is part of Department of Health and Human Services.)[179] The FDA stated it is also responding to this threat by:
- working with other government agencies and manufacturers on a series of issues related to antiviral medications.
- growing the 2009 H1N1 flu virus and preparing to make vaccine seed lots, which may be used eventually to produce a safe and effective vaccine.
- helping to prepare reagents needed for vaccine production and coordinating closely with other public health agencies for clinical development and testing.
- accelerating access to new diagnostic tools for this 2009 H1N1 flu virus[180]
On May 6, 2009, the FDA announced that it had approved a new manufacturing facility for seasonal flu vaccine, owned by Sanofi Pasteur, which could also be used for manufacturing a vaccine for the new H1N1 flu strain.[181] The FDA also issued a warning for consumers to be wary of products claiming to cure or prevent swine flu.[182]

0 deaths
1+ deaths
5+ deaths
20+ deaths
Other federal agencies[edit]
- Department of Homeland Security
Secretary Napolitano stated that DHS is the principal federal office for incidents such as the current H1N1 flu outbreak, and “Under that role, we have been leading a true collaborative effort.”[183] The Department of Homeland Security has a document, dated November 1, 2005, entitled “National Strategy for Pandemic Influenza”, detailing planning for potential pandemics. https://web.archive.org/web/20090507013213/http://www.pandemicflu.gov/plan/federal/pandemic-influenza.pdf
- State Department
The State Department suggested travelers to Mexico stay alert and comply with guidance from Mexican public health officials, but did not impose any travel restrictions on US citizens to Mexico.[184] However, the State Department did recommend US citizens avoid non-essential travel to Mexico.[185]
- Department of Agriculture
The Department of Agriculture (USDA) reported no swine in the US have been infected so far, but the USDA is monitoring swine across the US for signs of infection.[186]
- Department of Commerce
The Department of Commerce sent a letter to Russia and China requesting that those countries lift their ban on American pork products.[187]
- Department of Defense
The Department of Defense (DOD) is monitoring the swine flu situation and has contingency plans to deal with such outbreaks.[188] As of May 7, 2009, the DOD reports 104 confirmed cases among Armed Forces personnel and their families. DOD maintains a daily summary and map.[189]
- Department of Education
The Department of Education is providing guidance to schools in the US affected by swine flu, as well as precautions to take.[190]
State and local[edit]
Schools closed in many states in response to local flu outbreaks. By April 30, 2009, 300 U.S. schools and school districts had announced closures in response to the outbreak, giving 169,000 students time off.[191] On May 4, 2009, about 533 schools in 24 states in the U.S. were closed, affecting about 330,000 students.[192] On September 25, 2009, 42 schools were closed in eight states as the second wave of the pandemic began.[193]
On May 5, Kathleen Sebelius stated in a CDC news conference that school closures for single confirmed cases of H1N1 influenza were unnecessary, but that parents keep their child home if he or she displays an influenza-like illness.[194]
Travel industry[edit]
Several US airlines waived fees for cancellations and flight changes.[195] At least one cruise line changed itinerary to avoid Mexican ports of call.[196]
Summary[edit]
[citation needed]
| 2009 |
A(H1N1) Outbreak and pandemic milestones |
| 28 March |
 First case in the US of what would later be identified as swine flu origin. First case in the US of what would later be identified as swine flu origin. |
| 21 April |
 First case confirmed in California. First case confirmed in California. |
| 23 April |
 First case confirmed in Texas. First case confirmed in Texas. |
| 25 April |
 First case confirmed in Kansas. First case confirmed in Kansas. |
 Community outbreaks confirmed in the United States. Community outbreaks confirmed in the United States. |
| 26 April |
 First case confirmed in New York. First case confirmed in New York. |
 First case confirmed in Ohio. First case confirmed in Ohio. |
| Acting HHS Secretary Charles E. Johnson declares 2009 H1N1 a Public Health Emergency[197][198] |
| 28 April |
 First case confirmed in Indiana. First case confirmed in Indiana. |
| 29 April |
 First non-US citizen death confirmed in Texas. First non-US citizen death confirmed in Texas. |
 First case confirmed in Nevada. First case confirmed in Nevada. |
 First case confirmed in Arizona. First case confirmed in Arizona. |
 First case confirmed in Maine. First case confirmed in Maine. |
 First case confirmed in Massachusetts. First case confirmed in Massachusetts. |
 First case confirmed in Michigan. First case confirmed in Michigan. |
| 30 April |
 First case confirmed in Nebraska. First case confirmed in Nebraska. |
 First case confirmed in South Carolina. First case confirmed in South Carolina. |
 First case confirmed in Minnesota. First case confirmed in Minnesota. |
 First case confirmed in Colorado. First case confirmed in Colorado. |
 First case confirmed in Virginia. First case confirmed in Virginia. |
 First case confirmed in Kentucky. First case confirmed in Kentucky. |
 First case confirmed in New Jersey. First case confirmed in New Jersey. |
| 1 May |
 First case confirmed in Florida. First case confirmed in Florida. |
 First case confirmed in Missouri. First case confirmed in Missouri. |
 First case confirmed in Connecticut. First case confirmed in Connecticut. |
 First case confirmed in Delaware. First case confirmed in Delaware. |
| 2 May |
 First case confirmed in New Mexico. First case confirmed in New Mexico. |
 First case confirmed in Utah. First case confirmed in Utah. |
 First case confirmed in New Hampshire. First case confirmed in New Hampshire. |
 First case confirmed in Rhode Island. First case confirmed in Rhode Island. |
 First case confirmed in Iowa. First case confirmed in Iowa. |
 First case confirmed in Wisconsin. First case confirmed in Wisconsin. |
 First case confirmed in Alabama. First case confirmed in Alabama. |
| 3 May |
 First case confirmed in Idaho. First case confirmed in Idaho. |
 First case confirmed in Pennsylvania. First case confirmed in Pennsylvania. |
 First case confirmed in Louisiana. First case confirmed in Louisiana. |
 First case confirmed in North Carolina. First case confirmed in North Carolina. |
 First case confirmed in Tennessee. First case confirmed in Tennessee. |
| 4 May |
 First case confirmed in Oregon. First case confirmed in Oregon. |
 First case confirmed in Georgia. First case confirmed in Georgia. |
 First case confirmed in Maryland. First case confirmed in Maryland. |
| 5 May |
 First case confirmed in Washington. First case confirmed in Washington. |
 First case confirmed in Oklahoma. First case confirmed in Oklahoma. |
 First case confirmed in Hawaii. First case confirmed in Hawaii. |
 First US citizen death confirmed in Texas. First US citizen death confirmed in Texas. |
| 6 May |
 First case confirmed in District of Columbia. First case confirmed in District of Columbia. |
| 7 May |
 First case confirmed in South Dakota. First case confirmed in South Dakota. |
| 8 May |
 First case confirmed in Vermont. First case confirmed in Vermont. |
 First case confirmed in Arkansas. First case confirmed in Arkansas. |
| 9 May |
 First death confirmed in Washington. First death confirmed in Washington. |
| 11 May |
 First case confirmed in Montana. First case confirmed in Montana. |
| 13 May |
 First case confirmed in North Dakota. First case confirmed in North Dakota. |
| 14 May |
 First death confirmed in Arizona. First death confirmed in Arizona. |
| 15 May |
 First case confirmed in Mississippi. First case confirmed in Mississippi. |
| 17 May |
 First death confirmed in New York. First death confirmed in New York. |
| 19 May |
 First death confirmed in Missouri. First death confirmed in Missouri. |
| 20 May |
 First death confirmed in Utah. First death confirmed in Utah. |
| 25 May |
 First death confirmed in Illinois. First death confirmed in Illinois. |
| 26 May |
 First case confirmed in Puerto Rico. First case confirmed in Puerto Rico. |
| 27 May |
 First case confirmed in Wyoming. First case confirmed in Wyoming. |
 First case confirmed in Alaska. First case confirmed in Alaska. |
| 2 June |
 First case confirmed in West Virginia. All 50 states have confirmed H1N1. First case confirmed in West Virginia. All 50 states have confirmed H1N1. |
 First death confirmed in Virginia. First death confirmed in Virginia. |
| 3 June |
 First death confirmed in Michigan. First death confirmed in Michigan. |
 First death confirmed in Connecticut. First death confirmed in Connecticut. |
| 4 June |
 First death confirmed in California. First death confirmed in California. |
| 5 June |
 First death confirmed in Pennsylvania. First death confirmed in Pennsylvania. |
 First death confirmed in Wisconsin. First death confirmed in Wisconsin. |
| 8 June |
 First death confirmed in Oregon. First death confirmed in Oregon. |
 First death confirmed in Oklahoma. First death confirmed in Oklahoma. |
| 15 June |
 First death confirmed in New Jersey. First death confirmed in New Jersey. |
 First death confirmed in Massachusetts. First death confirmed in Massachusetts. |
 First death confirmed in Minnesota. First death confirmed in Minnesota. |
| 16 June |
 First death confirmed in Florida. First death confirmed in Florida. |
 First death confirmed in Rhode Island. First death confirmed in Rhode Island. |
 First case confirmed in US Virgin Islands. First case confirmed in US Virgin Islands. |
| 23 June |
 First death confirmed in Maryland. First death confirmed in Maryland. |
| 24 June |
 First death confirmed in North Carolina. First death confirmed in North Carolina. |
| 26 June |
 First case confirmed in American Samoa. First case confirmed in American Samoa. |
| 29 June |
 First death confirmed in Hawaii. First death confirmed in Hawaii. |
| 1 July |
 First case confirmed in Guam. First case confirmed in Guam. |
| 6 July |
 First death confirmed in Ohio. First death confirmed in Ohio. |
 First death confirmed in Nevada. First death confirmed in Nevada. |
| 10 July |
 First death confirmed in Indiana. First death confirmed in Indiana. |
 First death confirmed in Georgia. First death confirmed in Georgia. |
| 15 July |
 First death confirmed in Nebraska. First death confirmed in Nebraska. |
 First death confirmed in Tennessee. First death confirmed in Tennessee. |
| 20 July |
 First death confirmed in Guam. First death confirmed in Guam. |
| 21 July |
 First case confirmed in Northern Mariana Islands. First case confirmed in Northern Mariana Islands. |
| 27 July |
 First death confirmed in Alaska. First death confirmed in Alaska. |
| 29 July |
 First death confirmed in Alabama. First death confirmed in Alabama. |
 First death confirmed in Colorado. First death confirmed in Colorado. |
| 3 August |
 First death confirmed in Iowa. First death confirmed in Iowa. |
| 6 August |
 First death confirmed in Kansas. First death confirmed in Kansas. |
 First death confirmed in Montana. First death confirmed in Montana. |
 First death confirmed in Mississippi. First death confirmed in Mississippi. |
| 8 August |
 First death confirmed in Arkansas. First death confirmed in Arkansas. |
| 10 August |
 First death confirmed in New Mexico. First death confirmed in New Mexico. |
| 13 August |
 First death confirmed in Maine. First death confirmed in Maine. |
 First death confirmed in Louisiana. First death confirmed in Louisiana. |
| 14 August |
 First case of Oseltamivir (Tamiflu) resistance confirmed. First case of Oseltamivir (Tamiflu) resistance confirmed. |
| 17 August |
 First death confirmed in New Hampshire. First death confirmed in New Hampshire. |
| 19 August |
 First death confirmed in Wyoming. First death confirmed in Wyoming. |
| 28 August |
 First death confirmed in South Carolina. First death confirmed in South Carolina. |
| 2 September |
 First death confirmed in US Virgin Islands. First death confirmed in US Virgin Islands. |
| 3 September |
 First death confirmed in Kentucky. First death confirmed in Kentucky. |
| 4 September |
 First death confirmed in West Virginia. First death confirmed in West Virginia. |
| 10 September |
 First Oseltamivir (Tamiflu) resistance spread from person to person confirmed. First Oseltamivir (Tamiflu) resistance spread from person to person confirmed. |
| 28 September |
 First death confirmed in Idaho. First death confirmed in Idaho. |
| 2 October |
 First death confirmed in South Dakota. First death confirmed in South Dakota. |
| 22 October |
 First death confirmed in Delaware. First death confirmed in Delaware. |
| 26 October |
 First death confirmed in North Dakota. First death confirmed in North Dakota. |
| 28 October |
President Obama declares H1N1 a National Emergency.[199] |
 First death confirmed in Vermont. All 50 states have confirmed H1N1 deaths. First death confirmed in Vermont. All 50 states have confirmed H1N1 deaths. |
| 4 November |
 First feline zoonosis confirmed in Iowa. First feline zoonosis confirmed in Iowa. |
| 19 November |
 First death confirmed in American Samoa. First death confirmed in American Samoa. |
 First feline death confirmed in Oregon. First feline death confirmed in Oregon. |
| 24 November |
 First double infection case confirmed in West Virginia. First double infection case confirmed in West Virginia. |























