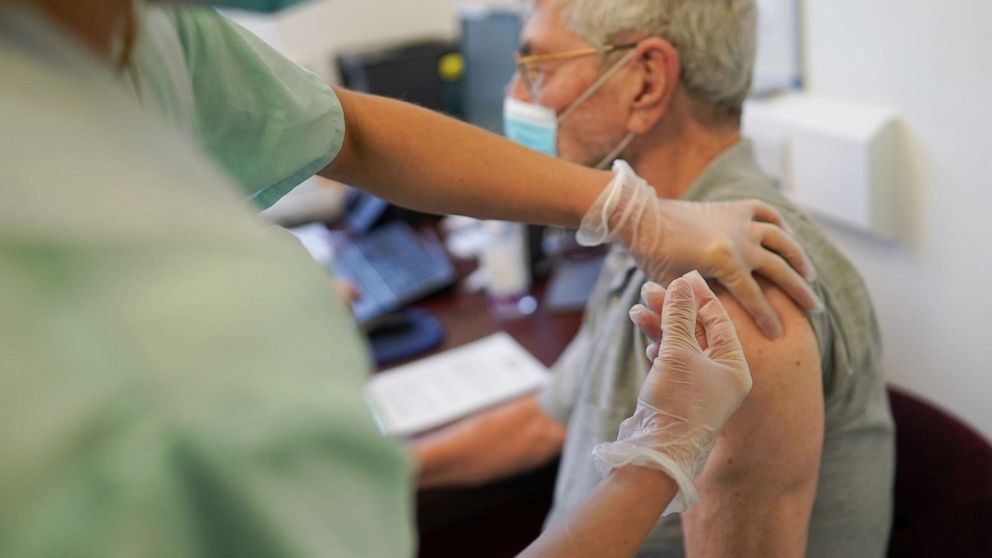
The US Centers for Disease Control and Prevention (CDC) has announced that it will begin screening travelers at airports for COVID and other viruses. Did you think the governments would actually let go of the power they usurped after COVID? The government turned a blind eye to the MILLIONS of illegals entering America from around the world. No one cared about what illnesses they brought with them. The program is intended to target Americans and international travelers who are here legally. Our freedom of movement is at risk for a variety of reasons, and this is merely another step toward the coming restrictions.
Not only does the government want to restrict our movement, but they also want our biological data. The Traveler-based Genomic Surveillance program collects nasal swab samples from volunteers at six major US international airports. The program began after COVID was released on the population, and they have only expanded their reach. This is not merely a government surveillance program as both Ginkgo Bioworks and XpresSpa Group, which are private and publicly traded organizations, have partnered with the CDC to collect data. The goal? Biosurveillance.
“TGS is a flexible, multimodal platform that consists of three complementary approaches of sample collection from arriving international travelers at U.S. airports, including voluntary nasal swabbing, aircraft wastewater, and airport wastewater sampling to enhance early detection of new SARS-CoV-2 variants and other pathogens, and fills gaps in global surveillance,” the various newsletters states. The government does not care about our drinking water (see: Flint, East Palestine, etc.). They could easily claim contamination and prevent planes from leaving. Also, I don’t know about you, but if I am traveling the last thing I want to do is stop for a medical assessment. This program will only remain voluntary for a limited time.
New York, JFK, San Francisco, Boston, and Washington DC, Dulles will be the first testing zones before this is rolled out to the entire country. “Enhancing surveillance capacity for global mass gathering/migration events,” has been listed as a main goal on the CDC’s website. “Migration events” is an interesting propaganda phrase. We have passports, visas, and other programs in place to track those coming and going. The migration event at the southern border has been of no concern since Biden entered the White House.
In other words, private companies are partnering with the CDC to provide the government with bio data to track everyone who is legally traveling to and from America. They want to keep track of their pawns and will collect our individual bio data to do so. They now have an excuse to prohibit us from traveling or restricting our freedom of movement. Taking it a step further, they believe they can program our DNA like code and control nature, but that is a topic for another time.